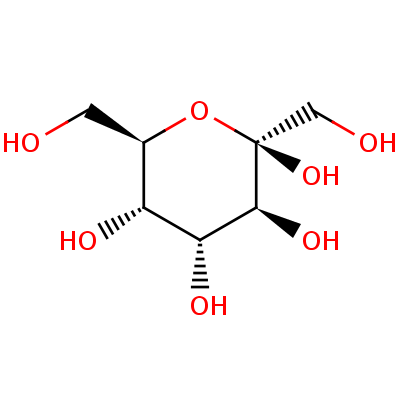
In natural science, a hexose is a monosaccharide with six carbon iotas, having the compound recipe C
6H
12O
6. Hexoses are arranged by useful gathering, with aldohexoses having an aldehyde at position 1, and ketohexoses having a ketone at position 2.
Aldohexoses
The aldohexoses have four chiral communities for an aggregate of 16 conceivable aldohexose stereoisomers (24). The D/L design is focused around the introduction of the hydroxyl at position 5, and does not allude to the course of optical action. The eight D-aldohexoses are:

Of these D-isomers, all with the exception of D-altrose are characteristically happening. L-Altrose, on the other hand, has been detached from strains of the bacterium Butyrivibrio fibrisolvens.
A memory helper for the aldohexoses is "All Altruists Gladly Make Gum in Gallon Tanks": allose, altrose, glucose, mannose, gulose, idose, galactose, talose. At the point when attracted this request, the Fischer projections of the D-aldohexoses take after an example. Allose has each of the four hydroxyl bunches on the right. At carbon 2, the hydroxyl gatherings substitute right-left. At carbon 3, the initial two are on the right, the following two are on the left, et cetera. At carbon 4, the initial four are on the right and the rest are on the left. At carbon 5, each of the eight D-aldohexoses have the hydroxyl assemble on the right.
This could be seen as twofold including to eight, where 0 stands for hydroxyl and 1 for hydrogen. So 0000 stands for D-Allose, 0001 stands for D-Altrose, 0010 stands for D-Glucose, 0011 stands for D-Mannose, 0100 stands for D-Gulose, 0101 stands for D-Idose, 0110 stands for D-Galactose and 0111 stands for D-Talose.
Cyclic hemiacetals
It has been known since 1926 that 6-carbon aldose sugars structure cyclic hemiacetals. The chart underneath demonstrates the hemiacetal structures for D-glucose and D-mannose.
The numbered carbons in the open-tie structures relate to the same numbered carbons in the hemiacetal structures. The shaping of the hemiacetal reasons carbon number 1, which is symmetric in the open-chain structure, to wind up awry in the cyclic adaptation. This implies that both glucose and mannose (and also the various aldohexoses) each one have two cyclic structures. In result, both of these exist in harmony with the open-chain structure. The open-chain structure, on the other hand, does not solidify. Subsequently the two cyclic structures get to be distinct when they are solidified. For instance, D-glucose structures an alpha gem that has particular turn of +112° and liquefying purpose of 146 °c, and additionally a beta precious stone that has particular pivot of +19° and softening purpose of 150 .
Ketohexoses
The ketohexoses have 3 chiral focuses and in this way eight conceivable stereoisomers (2
3). Of these, just the four D-isomers are known to happen regularly:
 A disaccharide or biose is the carbohydrate formed when two monosaccharides
undergo a condensation reaction which involves the elimination of a
small molecule, such as water, from the functional groups only. Like
monosaccharides, disaccharides form an aqueous solution when dissolved
in water. Three common examples are sucrose, lactose, and maltose.
A disaccharide or biose is the carbohydrate formed when two monosaccharides
undergo a condensation reaction which involves the elimination of a
small molecule, such as water, from the functional groups only. Like
monosaccharides, disaccharides form an aqueous solution when dissolved
in water. Three common examples are sucrose, lactose, and maltose. Depending on the monosaccharide constituents, disaccharides are sometimes crystalline, sometimes water-soluble, and sometimes sweet-tasting and sticky-feeling.
Depending on the monosaccharide constituents, disaccharides are sometimes crystalline, sometimes water-soluble, and sometimes sweet-tasting and sticky-feeling.